Fall SAVY 2018, Day 1 – Hydrology 101 (1st/2nd)
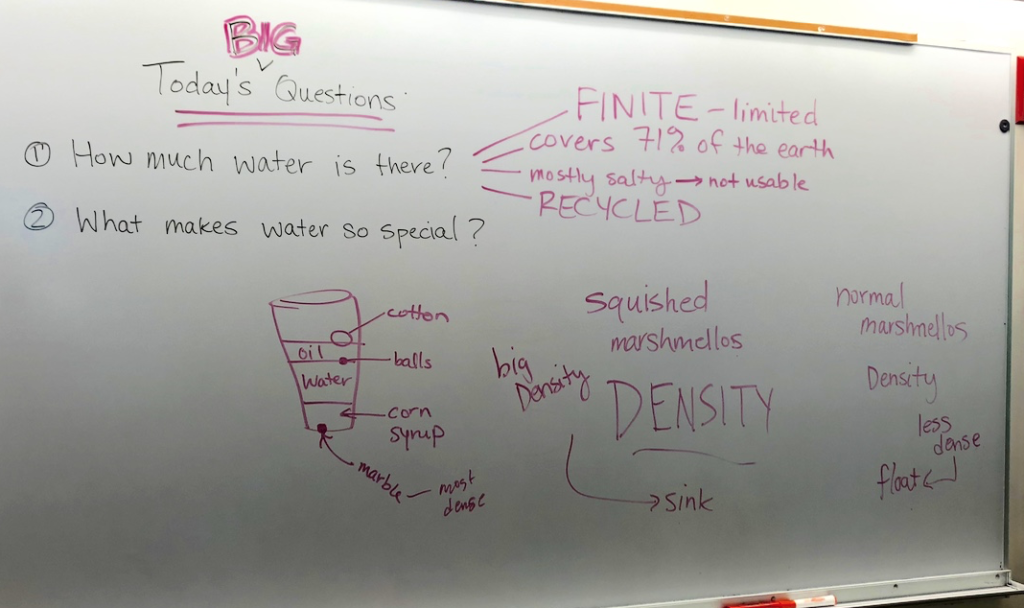
How much water is there? On Earth, there are more than 326 million trillion gallons of water, which covers 71% of the globe. That may sound like a lot of water, but less than 3% of all this water is freshwater. And of all the freshwater, more than two-thirds is locked up in ice caps and glaciers. So water is FINITE, or limited. The same water we drink today is the water that quenched the dinosaurs’ thirst! Water molecules are RECYCLED again and again–flowing through watersheds, evaporating to the upper atmosphere, condensing into clouds, and raining down again. It is an amazing balance that supports all life on Earth!
But what is it that makes water so special? The matter that makes up water has some pretty phenomenal properties. The hydrogen and oxygen atoms that make up each water molecule create strong bonds that govern the way water behaves. The observable cohesion, adhesion, solubility, and liquid density properties that make water so unique also have completely shaped our world. Imagine our world if water properties were slightly different. Perhaps there would be no iced tea…?
Students, also consider trying this trick with water, pepper, and soap. I bet you might be able to make some connections to our Magic Soap (and penny) experiment in class.
I’m really looking forward to next week’s field trip to the Earth and Environmental Sciences laboratories, where we will play with stream, aquifer, and glacier models! Get ready to get wet (and dirty)!
Best,
Chelsea
Learning About the Water Cycle